Luspatercept-aamt (REBLOZYL®) is a preferred, first-line treatment option for symptomatic anemia in RS- and RS+ LR-MDS as recommended by the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)1*
EFFICACY and safety: primary endpoint
Superior rates of response: Hgb increase and transfusion independence2
Primary composite endpoint: Hgb increase ≥1.5 g/dL and RBC-TI for at least 12 weeks2

*For patients with IPSS-R very low-, low-, or intermediate-risk MDS with non-del(5q) ± other cytogenetic abnormalities and with RS <15% (or RS <5% with SF3B1 mutation) with sEPO ≤500 mU/mL or with non-del(5q) ± other cytogenetic abnormalities with RS ≥15% (or RS ≥5% with an SF3B1 mutation).1
Key secondary endpoints included HI-E per IWG ≥8 weeks (Weeks 1-24): REBLOZYL 74.1% (109/147), epoetin alfa 51.3% (n=79/154); RBC-TI for 24 weeks (Weeks 1-24): REBLOZYL 47.6% (n=70/147), epoetin alfa 29.2% (n=45/154); and RBC-TI for ≥12 weeks (Weeks 1-24): REBLOZYL 66.7% (n=98/147), epoetin alfa 46.1% (n=71/154).2

It's not if, but when—
80% of all patients receiving REBLOZYL had their dose increased at least once.3

Patient population and study design
REBLOZYL was studied head-to-head vs an ESA2
COMMANDS: A Phase 3 open-label, randomized, active-controlled, head-to-head trial of REBLOZYL vs epoetin alfa in anemia due to LR-MDS in ESA-naive patients2,4
Select graphic view or text view to explore data

Patient Population (N=356)2,4,5
- Adults ≥18 years of age
- IPSS-R very low-, low-, or intermediate-risk MDS
- RS-positive and RS-negative
- ESA-naive
- Endogenous sEPO <500 U/L
- Requiring RBC transfusion for Hgb ≤9 g/dL with symptoms or Hgb ≤7 g/dL without symptoms and 2 to 6 units of RBCs within 8 weeks prior to randomization
- Excluded: patients with del(5q) and those previously treated with disease-modifying agents or HMAs
Randomized 1:1
REBLOZYL2
1 mg/kg SC Q3W, with titration up to max
1.75 mg/kg if needed to achieve a response
(n=178)
EPOETIN ALFA2,4†
450 IU/kg SC QW max total dose 40K IU,
with titration up if needed to 1050 IU/kg
max total dose 80K IU
(n=178)
Both treatments were dose modified targeting Hgb between 10-12 g/dL and TI6
All patients received BSC, which included RBC transfusions as needed2
Composite primary endpoint2
For any consecutive 12 week period during Weeks 1 to 24:
- RBC-TI
and - Mean imporvement in Hgb by at least 1.5 g/dL
Key secondary endpoints2
- HI-E response per IWG ≥8 weeks
(Weeks 1-24) - RBC-TI for 24 weeks (Weeks 1-24)
- RBC-TI for ≥12 weeks (Weeks 1-24)
Other secondary endpoints2,4
- Mean Hgb increase ≥1.5 g/dL (Weeks 1-24)
- Duration of RBC-TI≥12 weeks (Weeks 1-EOT)
- Time to first RBC transfusion (Week 1-EOT)
- Hgb change from baseline over 24 weeks (Weeks 1-24)
†>90% of study participants were outside of the United States and used a non-US-licensed epoetin alfa product. Direct comparisons between REBLOZYL and US-licensed epoetin alfa product have not been established.2
Interim analysis of COMMANDS reported.2
Baseline disease characteristics
Balanced across study arms events in pivotal Phase 3 COMMANDS trial2
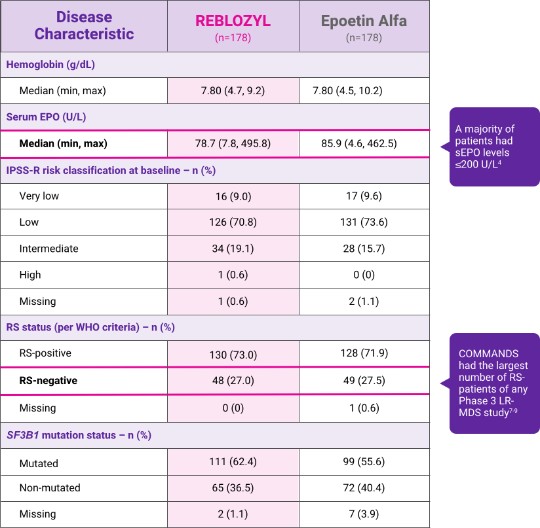
Baseline Disease Characteristics table for REBLOZYL (n=178) and Epoetin Alfa (n=178)
Hemoglobin (g/dL)
- Median (min, max): REBLOZYL 7.80 (4.7, 9.2) and Epoetin Alfa 7.80 (4.5, 10.2)
Serum EPO (U/L)
- Median (min, max): REBLOZYL 78.7 (7.8, 495.8) and Epoetin Alfa 85.9 (4.6, 462.5)
A majority of patients had sEPO levels ≤200 U/L4
IPSS-R risk classification at baseline — n(%)
- Very low: REBLOZYL 16 (9.0) and Epoetin Alfa 17 (9.6)
- Low: REBLOZYL 126 (70.8) and Epoetin Alfa 131 (73.6)
- Intermediate: REBLOZYL 34 (19.1) and Epoetin Alfa 28 (15.7)
- High: REBLOZYL 1 (0.6) and Epoetin Alfa 0 (0)
- Missing: REBLOZYL 1 (0.6) and Epoetin Alfa 2 (1.1)
RS status (per WHO criteria) — n (%)
- RS-positive REBLOZYL 130 (73.0) and Epoetin Alfa 128 (71.9)
- RS-negative REBLOZYL 48 (27.0) and Epoetin Alfa 49 (27.5)
COMMANDS had the largest number of RS- patients of any Phase 3 LR-MDS study7,9
- Missing: REBLOZYL 0 (0) and Epoetin Alfa 1 (0.6)
SF3B1 mutation status
- Mutated: REBLOZYL 111 (62.4) and Epoetin Alfa 99 (55.6)
- Non-mutated: REBLOZYL 65 (36.5) and Epoetin Alfa 77 (40.4)
- Missing: REBLOZYL 2(1.1) and Epoetin Alfa 7 (3.9)
BSC=best supportive care; CI=confidence interval; EOT=end of treatment; EPO=erythropoietin; ESA=erythropoiesis-stimulating agent; Hgb=hemoglobin; HI-E=hematologic improvement-erythroid; HMA=hypomethylating agent; IPSS-R=Revised International Prognostic Scoring System; IWG=International Working Group; LR-MDS=lower-risk myelodysplastic syndromes; MDS=myelodysplastic syndromes; NCCN=National Comprehensive Cancer Network; QW=once a week; Q3W=once every 3 weeks; RBC=red blood cell; RBC-TI=red blood cell transfusion independence; RS=ring sideroblast; SC=subcutaneous; sEPO=serum erythropoietin; TI=transfusion independence; WHO=World Health Organization.
References: 1. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Myelodysplastic Syndromes V.1.2025. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed November 18, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. 2. REBLOZYL [US Prescribing Information]. Summit, NJ: Celgene Corporation; 2024. 3. Santini V, Zeidan AM, Platzbecker U, et al. Clinical benefit of luspatercept treatment in transfusion-dependent, erythropoiesis-stimulating agent-naive patients with Very low-, Low- or Intermediate-risk myelodysplastic syndromes in the COMMANDS trial. Poster presented at: European Hematology Association (EHA) Hybrid Congress. June 13-16, 2024. Madrid, Spain. 4. Platzbecker U, Della Porta MG, Santini V, et el. Efficacy and safety of luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes (COMMANDS): interim analysis of a phase 3, open-label, randomised controlled trial. Lancet. 2023;402(10399):373-385. 5. Platzbecker U, Della Porta MG, Santini V, et el. Efficacy and safety of luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes (COMMANDS): interim analysis of a phase 3, open-label, randomised controlled trial (supplementary appendix). Lancet. 2023; published online June 10, 2023. https://doi.org/10.1016/S0140-6736(23)00874-7 6. Data on file. BMS-REF-00730-2007. Princeton, NJ: Bristol-Myers Squibb Company; 2024. 7. Fenaux P, Santini V, Spiriti MAA, et al. A phase 3 randomized, placebo-controlled study assessing the efficacy and safety of epoetin-a in anemic patients with low-risk MDS. Leukemia. 2018;32(12):2648-2658. 8. Greenberg PL, Tuechler H, Schanz J, et al. Revised International Prognostic Scoring System for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465. 9. Garcia-Manero G, Platzbecker U, Santini V, et al. Efficacy and safety of luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive patients with transfusion-dependent lower-risk myelodysplastic syndromes: full analysis of the COMMANDS trial. Presented at: American Society of Hematology (ASH) Annual Meeting and Exposition. December 9-12, 2023. San Diego, CA. Abstract 193.

