Demographic |
REBLOZYL + BSC
|
Placebo + BSC
|
|---|---|---|
| Age, years Median (min, max) |
30.0 (18, 66) | 30.0 (18, 59) |
| Baseline transfusion burden 12 weeks prior to randomization, units/12 weeks Median (min, max) |
6.12 (3, 14) | 6.27 (3, 12) |
| Beta-thalassemia gene mutation grouping, n (%) β0/β0 Non-β0/β0 Missinga |
68 (30.4) 155 (69.2) 1 (0.4) |
35 (31.3) 77 (68.8) 0 |
| Baseline serum ferritin level, μg/L N Median (min, max) |
220 1441.25 (88, 6400) |
111 1301.50 (136, 6400) |
| Splenectomy, n (%) Yes No |
129 (57.6) 95 (42.4) |
65 (58) 47 (42) |
| Sex, n (%) Male Female |
92 (41.1) 132 (58.9) |
49 (43.7) 63 (56.3) |
| Beta-thalassemia diagnosis, n (%) β-thalassemia HbE/ β-thalassemia β-thalassemia combined with α-thalassemia Missinga |
174 (77.7) 31 (13.8) 18 (8) 1 (0.4) |
83 (74.1) 21 (18.8) 8 (7.1) 0 |
PATIENT OUTCOMES: REBLOZYL EFFICACY
REBLOZYL provided substantial clinical benefit by reducing RBC transfusion burden1,2
The efficacy of REBLOZYL in patients with beta-thalassemia was demonstrated in the BELIEVE trial
Study design: REBLOZYL was studied in the pivotal Phase 3 BELIEVE trial of 336 adult patients with beta-thalassemia requiring regular RBC transfusions (6-20 RBC units per 24 weeks) with no transfusion-free period greater than 35 days during that period who were randomized 2:1 to REBLOZYL (n=224) or placebo (n=112). In BELIEVE, REBLOZYL was administered subcutaneously once every 3 weeks as long as a reduction in transfusion requirement was observed or until unacceptable toxicity. Patients were able to receive BSC as needed, including: RBC transfusions; iron-chelating agents; use of antibiotic, antiviral, and antifungal therapy; and nutritional support. The exclusion criteria for this trial included HbS/beta-thalassemia or alpha-thalassemia; major organ damage (liver, heart, or lung disease, or renal insufficiency); recent deep vein thrombosis or stroke; or recent use of ESA, immunosuppressant, or hydroxyurea therapy. At 48 weeks, patients could cross over to REBLOZYL as part of the long-term follow-up.1,3
BELIEVE patient population and study design1,2
All patients in the pivotal Phase 3 BELIEVE trial received regular RBC transfusions1
BELIEVE baseline disease characteristics1,2
Primary endpoint: RBC-TI ≥33% reduction from baseline in transfusion burden from Weeks 13 to 241


KEY SECONDARY ENDPOINTS
Clinically meaningful reductions in transfusion burden were seen with REBLOZYL1


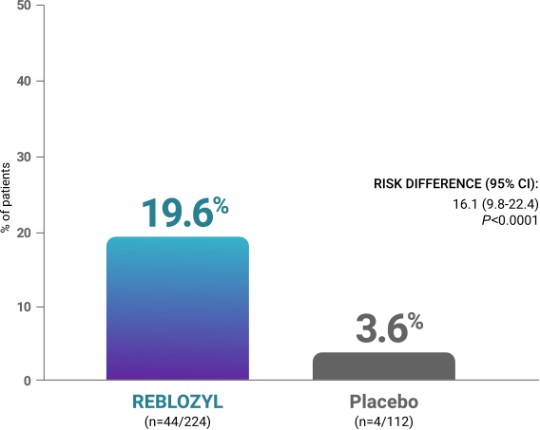



Additional analyses
Reductions in transfusion burden from baseline during any consecutive 24-week period with REBLOZYL2,3*
Time to response and total cumulative duration of response at 48 weeks3
BSC=best supportive care; CI=confidence interval; ESA=erythropoiesis-stimulating agent; HbE=hemoglobin E; HbS=hemoglobin S; RBC=red blood cell; RBC-TI=red blood cell transfusion independence; SC=subcutaneous.
Learn about the safety of REBLOZYL
References: 1. REBLOZYL [US Prescribing Information]. Summit, NJ: Celgene Corporation; 2023. 2. Cappellini MD, Viprakasit V, Taher AT, et al. A phase 3 trial of luspatercept in patients with transfusion-dependent β-thalassemia. N Engl J Med. 2020;382(13):1219-1231. 3. Data on file. Celgene Corporation. Summit, New Jersey.


