Demographic
|
REBLOZYL(n=153) |
Placebo(n=76) |
|---|---|---|
| Age, years Median (min, max) |
71.0 (40, 95) | 72.0 (26, 91) |
| Time since original MDS diagnosis,a months Median (min, max) |
44.0 (3, 421) | 36.1 (4, 193) |
| Serum EPO (U/L categories,b n (%) <200 200 to 500 >500 Missing |
88 (57.5) 43 (28.1) 21 (13.7) 1 (0.7) |
50 (65.8) 15 (19.7) 11 (14.5) 0 |
| RBC transfusion/8 weeks over 16 weeks, n (%) <4 units ≥4 and <6 units ≥6 units |
46 (30.1) 41 (26.8) 66 (43.1) |
20 (26.3) 23 (30.3) 33 (43.4) |
| Diagnosis per WHO 2016 criteria,c n (%) MDS-RSd MDS/MPN-RS-T Other |
135 (88.2) 14 (9.2) 4 (2.6) |
65 (85.5) 9 (11.8) 2 (2.6) |
| SF3B1, n (%) Mutated Nonmutated Missing |
138 (93.2) 10 (6.8) 0 |
64 (86.5) 10 (13.5) 1 (1.3) |
| IPSS-R classification risk category, n (%) Very low Low Intermediate High |
18 (12) 109 (71) 25 (16) 1 (1) |
6 (8) 57 (75) 13 (17) 0 |
PATIENT OUTCOMES: REBLOZYL EFFICACY IN SECOND-LINE MDS PATIENTS
With REBLOZYL, you can now achieve transfusion independence AND hematologic improvement1
Study design: REBLOZYL was studied in the pivotal Phase 3 MEDALIST trial of 229 patients with IPSS-R very low-, low-, or intermediate-risk MDS who have ring sideroblasts and require RBC transfusions (≥2 RBC units/8 weeks) who were randomized 2:1 to REBLOZYL (n=153) or placebo (n=76). Patients were required to have had an inadequate response to prior treatment with an ESA, be intolerant of ESAs, or be ineligible for ESAs (serum EPO >200 U/L). MEDALIST excluded patients with del(5q) MDS, white blood cell count >13 Gi/L, neutrophils <0.5 Gi/L, platelets <50 Gi/L, or with prior use of a disease-modifying agent for treatment of MDS. REBLOZYL was administered 1 mg/kg subcutaneously every 3 weeks.1
EPO=erythropoietin; ESA=erythropoiesis-stimulating agent; IPSS-R=Revised International Prognostic Scoring System; MDS=myelodysplastic syndromes; RBC=red blood cell.
MEDALIST patient population and study design1,2
MEDALIST baseline disease characteristics1,3,4
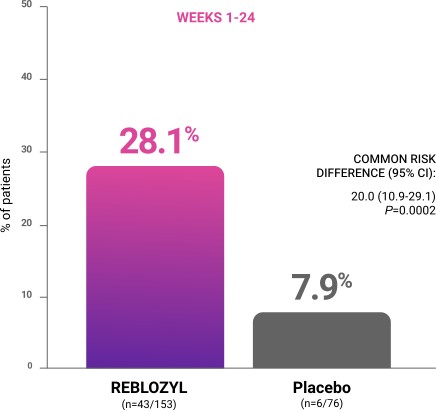
Primary endpoint: RBC-TI ≥8 weeks during weeks 1 to 241


CI=confidence interval; RBC-TI=red blood cell transfusion independence.
60%
(n=35/38)
of responders required at least 1 dose increase to achieve RBC-TI2
Key secondary endpoints: RBC-TI ≥12 weeks1
Additional important clinical data1
For appropriate patients who have failed an ESA…
Switch to REBLOZYL early, while transfusion burden is low
RBC-TI ≥8 weeks during Weeks 1 to 24 by diagnosis and baseline transfusion burden in MEDALIST1



80%
of patients with
low transfusion
burden become
transfusion-
independent
with REBLOZYL
treatment1
aIncludes MDS-EB-1, MDS-EB-2, and MDS-U. bIncludes patients who received 3.5 units. cIncludes patients who received 5.5 units.
MDS-EB-1=myelodysplastic syndromes with excess blasts (5%-9% in the bone marrow or 2%-4% in the blood); MDS-EB-2=myelodysplastic syndromes with excess blasts (10%-19% in the bone marrow or 5%-19% in the blood); MDS-U=myelodysplastic syndromes, unclassifiable.
Additional analysis: Modified hematologic improvement-erythroid (mHI-E) was assessed in patients receiving REBLOZYL2
mHI-E was defined per the IWG criteria as the proportion of patients who met mHI-E criteria sustained over any consecutive 56-day (8-week) period2:
- For patients with baseline RBC transfusion burden of at least 4 units/8 weeks: response was defined as a reduction in RBC transfusion burden of ≥4 RBC units/8 weeks
- For patients with baseline RBC transfusion burden of less than 4 units/8 weeks: response was defined as a mean Hgb increase of ≥1.5 g/dL/8 weeks in the absence of transfusions for at least 8 weeks
Hgb=hemoglobin; IWG=International Working Group.
Patients achieving mHI-E in MEDALIST2
Weeks 1-24 |
Weeks 1-48 |
|||
|---|---|---|---|---|
REBLOZYL(n=153) |
Placebo(n=76) |
REBLOZYL(n=153) |
Placebo(n=76) |
|
| Modified hematologic improvement- erythroid (mHI-E) |
52.9% (81/153) |
11.8% (9/76) |
58.8% (90/153) |
17.1% (13/76) |
| RBC transfusion reduction of ≥4 units/8 weeksa |
48.6% (52/107) |
14.3% (8/56) |
54.2% (58/107) |
21.4% (12/56) |
| Hgb increase of ≥1.5 g/dL for 8 weeksb |
63.0% (29/46) |
5.0% (1/20) |
69.6% (32/46) |
5.0% (1/20) |
aPercentage based on number of patients with baseline RBC transfusion burden of ≥4 units/8 weeks (n=107 in the REBLOZYL arm).
bPercentage based on number of patients with baseline RBC transfusion burden of <4 units/8 weeks (n=46 in the REBLOZYL arm).
Analysis limitations
- The mHI-E analysis is a broader analysis than the primary endpoint of transfusion independence, and included patients who did not meet the primary endpoint, but2:
- Achieved transfusion reduction of ≥4 units over 8 weeks (with higher baseline transfusion burden)
- Achieved an Hgb increase of ≥1.5 g/dL for 8 weeks in the absence of transfusions (with lower baseline transfusion burden)
- Patients may have experienced multiple periods of response intermittently between periods without response over the 24-week assessment period and extension phase through 25 to 48 weeks2
- All patients in both arms were eligible to receive BSC, which included RBC transfusions as needed1
- These exploratory analyses should not be interpreted to determine treatment difference between arms in these select endpoints because of potential selection bias, insufficient sample size, and a higher probability of making a false positive finding
BSC=best supportive care.
Explore the safety of REBLOZYL
References: 1. REBLOZYL [US Prescribing Information]. Summit, NJ: Celgene Corporation; 2023. 2. Data on file. Celgene Corporation. Summit, New Jersey. 3. Fenaux P, Platzbecker U, Mufti GJ, et al. Luspatercept in patients with lower-risk myelodysplastic syndromes. N Engl J Med. 2020;382(2):140-151. 4. Fenaux P, Platzbecker U, Mufti GJ, et al. Luspatercept in patients with lower-risk myelodysplastic syndromes. N Engl J Med. 2020;382(suppl):1-37.