SCENARIO |
REBLOZYL
|
|---|---|
| Predose Hgb is ≥11.5 g/dL is in the absence of transfusions |
|
| Increase in Hgb >2 g/dL within 3 weeks in the absence of transfusions and: | |
| Current dose to 1.75 mg/kg |
|
| Current dose to 1.33 mg/kg |
|
| Current dose to 1 mg/kg |
|
| Current dose to 0.8 mg/kg |
|
| Current dose to 0.6 mg/kg |
|
DOSING AND ADMINISTRATION: REBLOZYL DOSING FOR ESA-NAIVE MDS PATIENTS
Help your patients achieve initial and subsequent responses with REBLOZYL dose increases
80% of all 1L patients receiving REBLOZYL required a dose increase at least once1*

Target hemoglobin increase and transfusion independence
58.8%† of patients treated with REBLOZYL achieved at least 1.5 g/dL increase in Hgb and at least 12 weeks TI vs 31.2%‡ of patients treated with epoetin alfa2

Evaluate your patient’s response before each dose and monitor hemoglobin, transfusion needs, and tolerability2

Escalate to a therapeutic dose to achieve initial and subsequent responses1
Pause or dose reduce as needed to manage adverse reactions and rapid Hgb rise1§
*Based on results of COMMANDS, a head-to-head trial vs epoetin alfa.2
†n=86/147; 95% CI: 50.1, 66.6.2
‡n=48/154; 95% CI: 24.0, 39.1.2
§In the absence of transfusion, if predose Hgb is ≥11.5 g/dL or if Hgb increases >2 g/dL within 3 weeks, interrupt or decrease dose.2

It's not if, but when—
most patients will need dose increases.1

Escalate to a therapeutic dose to achieve initial and subsequent responses
Select graphic view or text view to explore data.
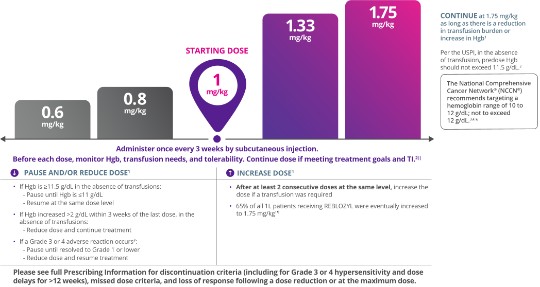
The starting dose is 1 mg/kg.
Lower doses available are 0.6 mg/kg and 0.8 mg/kg. Higher doses available are 1.33 mg/kg and 1.75 mg/kg.
Administer once every 3 weeks by subcutaneous injection.
Before each dose, monitor Hgb, transfusion needs, and tolerability. Continue dose if meeting treatment goals and TI.2‖
Per the USPI, in the absence of transfusion, predose Hgb should not exceed 11.5 g/dL.2
The National Comprehensive Cancer Network® (NCCN®) recommends targeting a hemoglobin range of 10 to 12 g/dL; not to exceed 12 g/dL.3**
- If Hgb is ≥11.5 g/dL in the absence of transfusions:
- Pause until Hgb is ≤11 g/dL
- Resume at the same dose level
- If Hgb increase >2 g/dL with 3 weeks of the last dose, in the absence of transfusions:
- Reduce dose and continue treatment
- If a Grade 3 or 4 adverse reaction occurs#
- Pause until resolved to Grade 1 or lower
- Reduce dose and resume treatment
- After at least 2 consecutive doses at the same level, increase the dose if a transfusion was required
- 65% of all 1L patients receiving REBLOZYL were eventually increased to 1.751§
Please see accompanying full Prescribing Information for discontinuation criteria (including for Grade 3 or 4 hypersensitivity and dose delays for >12 weeks), missed dose criteria, and loss of response following a dose reduction or at the maximum dose.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
‖If transfusion occurred, use pretransfusion Hgb before evaluation.2
¶Based on the results of COMMANDS, a head-to-head trial vs epoetin alfa.2
#Grade 1 is mild, Grade 2 is moderate, Grade 3 is severe, and Grade 4 is life-threatening.2
**For patients with symptomatic anemia due to LR-MDS.3
Some patients in COMMANDS required a treatment interruption or dose reduction
For super-responders with predose hemoglobin >11.5 g/dL or rapid hemoglobin rise1:
For adverse reactions1:
SCENARIO |
REBLOZYL
|
|---|---|
| Grade 3 or 4 hypersensitivity reactions†† |
|
| Other Grade 3 or 4 adverse reactions†† |
|
††Grade 1 is mild, Grade 2 is moderate, Grade 3 is severe, and Grade 4 is life-threatening.
‡‡Per dose reductions in table above.
Dose modifications when administering REBLOZYL1

Dose increases in the event of loss of response1
- If, upon dose reduction, the patient loses response (ie, requires a transfusion) or Hgb concentration drops by 1 g/dL or more in 3 weeks in the absence of transfusion, increase the dose by 1 dose level
- Wait a minimum of 6 weeks between dose increases
- Dose increases to 1.33 mg/kg and subsequently to 1.75 mg/kg may occur at any time during treatment after patients have received at least 2 consecutive doses at the prior lower dose level
- Do not increase the dose more frequently than every 2 consecutive doses (6 weeks) or beyond the maximum dose of 1.75 mg/kg

Discontinue treatment if no clinical benefit is observed1
- Discontinue REBLOZYL if no increase in Hgb or reduction in RBC transfusion burden from baseline after at least 3 consecutive doses (9 weeks) at 1.75 mg/kg or if unacceptable toxicity occurs at any time
If a planned administration of REBLOZYL is delayed or missed1
- Administer REBLOZYL as soon as possible and continue dosing as prescribed, with at least 3 weeks between doses
ESA=erythropoiesis-stimulating agent; Hgb=hemoglobin; MDS=myelodysplastic syndromes; RBC=red blood cell; RBCT=red blood cell transfusion; RBC-TI=red blood cell transfusion independence.
References: 1. Santini V, Zeidan AM, Platzbecker U, et al. Clinical benefit of luspatercept treatment in transfusion-dependent, erythropoiesis-stimulating agent-naive patients with Very low-, Low- or Intermediate-risk myelodysplastic syndromes in the COMMANDS trial. Poster presented at: European Hematology Association (EHA) Hybrid Congress. June 13-16, 2024. Madrid, Spain. 2. REBLOZYL [US Prescribing Information]. Summit, NJ: Celgene Corporation; 2024. 3. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Myelodysplastic Syndromes V.1.2025. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed November 18, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.

