DOSING AND ADMINISTRATION: ADMINISTERING REBLOZYL
REBLOZYL is administered subcutaneously (SC), once every 3 weeks1
REBLOZYL is available in 2 vial sizes (25 mg and 75 mg)
Instructions for administering REBLOZYL1
REBLOZYL should be reconstituted and administered by a healthcare professional
Prior to injection, allow solution to reach room temperature for a more comfortable injection.
Step 1: Verify correct dose for the patient
- Calculate the exact total dosing volume of 50 mg/mL solution required for the patient
Step 2: Plan and prepare for injection
- Slowly withdraw the dosing volume of the reconstituted REBLOZYL solution from the single-dose vial(s) into a syringe
- Divide doses requiring larger reconstituted volumes (ie, >1.2 mL) into separate, similar-volume injections and inject into separate sites
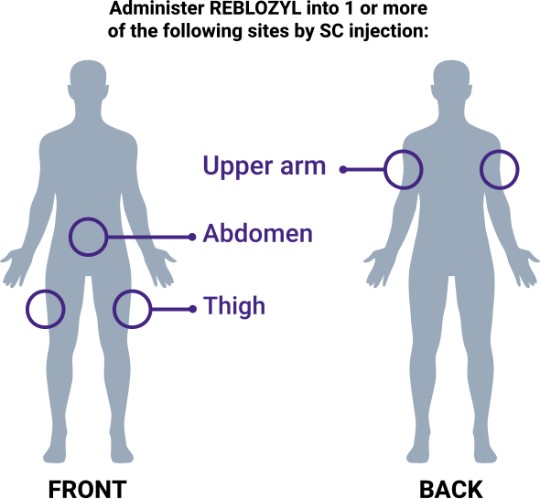
Step 3: Administer subcutaneously
- If multiple injections are required, use a new syringe and needle for each SC injection
NOTE: Discard any unused portion. Do not pool unused portions from the vials. Do not administer more than 1 dose from a vial. Do not mix with other medications.
Learn how to reconstitute REBLOZYL before administration
Reference: 1. REBLOZYL [US Prescribing Information]. Summit, NJ: Celgene Corporation; 2024.

